The U.S. Food and Drug Administration on Friday has for the first time approved the use of a respiratory syncytial virus (RSV) vaccine for people in their 50s who are at increased risk for the illness.
from Pfizer and Moderna, are already approved for use in adults 60 and older, since age brings higher risk from RSV disease. "A systematic review of studies in the US showed that RSV is estimated to cause 42,000 hospitalizations each year in adults aged 50-64 years old," GSK said in a company statement released Friday. are expected to meet in June and October, at which time they might also sign off on the use of Arexvy in people in their 50s.
GSK's latest trial data failed to reveal any "concerning" trends of GBS cases in the 50-to-59 age group, Dr. Phil Dormitzer, senior vice president and global head of"The risk of GBS tends to go up, it's another one of those risks that tends to go up, with age. But there's nothing to indicate that there's any particular risk of GBS," he said.
"This is a really key question," he said. "People originally anticipated it might have to be an every year immunization. But then we found the duration of protection actually lasts for more than one season, clearly."
United States Latest News, United States Headlines
Similar News:You can also read news stories similar to this one that we have collected from other news sources.
 FDA approves first RSV vaccine for at-risk adults in their 50sThe RSV vaccine by GSK was previously approved only for adults 60 and older.
FDA approves first RSV vaccine for at-risk adults in their 50sThe RSV vaccine by GSK was previously approved only for adults 60 and older.
Read more »
 Moderna's RSV Vaccine Approved by FDAFor the first time, an mRNA vaccine has been approved for an indication other than COVID-19. The vaccine will join a couple other immunizations currently on the market for respiratory syncytial virus.
Moderna's RSV Vaccine Approved by FDAFor the first time, an mRNA vaccine has been approved for an indication other than COVID-19. The vaccine will join a couple other immunizations currently on the market for respiratory syncytial virus.
Read more »
 FDA approves Moderna's RSV vaccine for seniors, the company's second-ever productThe decision is a win for Moderna, which needs another revenue source amid plunging demand for its Covid vaccine, its only commercially available product.
FDA approves Moderna's RSV vaccine for seniors, the company's second-ever productThe decision is a win for Moderna, which needs another revenue source amid plunging demand for its Covid vaccine, its only commercially available product.
Read more »
 FDA approves Moderna's RSV vaccine for seniors, the company's second-ever productThe decision is a win for Moderna, which needs another revenue source amid plunging demand for its Covid vaccine, its only commercially available product.
FDA approves Moderna's RSV vaccine for seniors, the company's second-ever productThe decision is a win for Moderna, which needs another revenue source amid plunging demand for its Covid vaccine, its only commercially available product.
Read more »
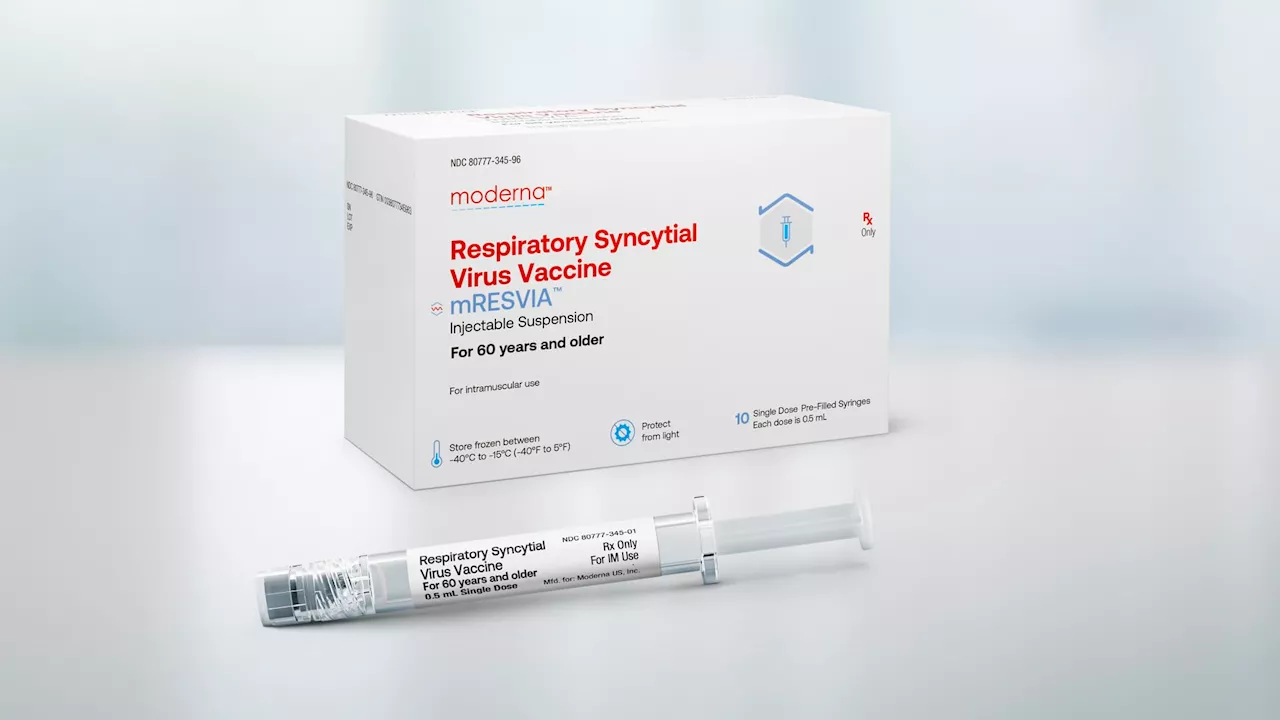 FDA approves Moderna's RSV vaccine for seniors, the company's second-ever productThe decision is a win for Moderna, which needs another revenue source amid plunging demand for its Covid vaccine, its only commercially available product.
FDA approves Moderna's RSV vaccine for seniors, the company's second-ever productThe decision is a win for Moderna, which needs another revenue source amid plunging demand for its Covid vaccine, its only commercially available product.
Read more »
 FDA Panel Gives Nod to Blood Test for Colon CancerA U.S. Food and Drug Administration advisory panel on Thursday recommended the approval of a new blood test that can spot colon cancer.
FDA Panel Gives Nod to Blood Test for Colon CancerA U.S. Food and Drug Administration advisory panel on Thursday recommended the approval of a new blood test that can spot colon cancer.
Read more »
