For the first time, an mRNA vaccine has been approved for an indication other than COVID-19. The vaccine will join a couple other immunizations currently on the market for respiratory syncytial virus.
The mRNA vaccine is approved for adults aged 60 years or older to prevent lower respiratory tract disease caused by RSV. It is the third vaccine to be approved for RSV in the past year after Arexvy from GSK and Abrysvo by Pfizer.
"The FDA approval of our second product, mRESVIA, builds on the strength and versatility of our mRNA platform," Stéphane Bancel, chief executive officer of Moderna, said in a."mRESVIA protects older adults from the severe outcomes of RSV infection. This approval is also the first time an mRNA vaccine has been approved for a disease other than COVID-19."
mRESVIA is a single-dose vaccine available in prefilled syringes, which the company says are designed to maximize ease of administration, saving vaccinators' time, and reducing the risk for administrative errors.The New England Journal of Medicine in December 2023. The study, conducted in approximately 37,000 adults aged 60 years or older in 22 countries, found a vaccine efficacy against RSV lower respiratory tract disease of 83.7% after a median 3.7 months of follow-up.
An additional longer-term analysis showed continued protection over 8.6 months median follow-up. No serious safety concerns were identified. The most reported adverse reactions were injection site pain, fatigue,Moderna has also filed for approval in multiple markets around the world, and says it expects mRESVIA to be available in the United States in time for the 2024-2025 respiratory virus season.
RSV Infection Respiratory Syncytial Virus (RSV) Infection Vaccine Vaccines Immunizations Vaccination Respiratory Tract Arthralgia Painful Joint Joint Pain Myalgia Muscle Pain Clinical Research Clinical Trials Clinical Studies Pre-Clinical Trial Double-Blind Study Double-Blind Studies Single-Blind Study Single-Blind Studies Fatigue
United States Latest News, United States Headlines
Similar News:You can also read news stories similar to this one that we have collected from other news sources.
 FDA approves Moderna's RSV vaccine for seniors, the company's second-ever productThe decision is a win for Moderna, which needs another revenue source amid plunging demand for its Covid vaccine, its only commercially available product.
FDA approves Moderna's RSV vaccine for seniors, the company's second-ever productThe decision is a win for Moderna, which needs another revenue source amid plunging demand for its Covid vaccine, its only commercially available product.
Read more »
 FDA approves Moderna's RSV vaccine for seniors, the company's second-ever productThe decision is a win for Moderna, which needs another revenue source amid plunging demand for its Covid vaccine, its only commercially available product.
FDA approves Moderna's RSV vaccine for seniors, the company's second-ever productThe decision is a win for Moderna, which needs another revenue source amid plunging demand for its Covid vaccine, its only commercially available product.
Read more »
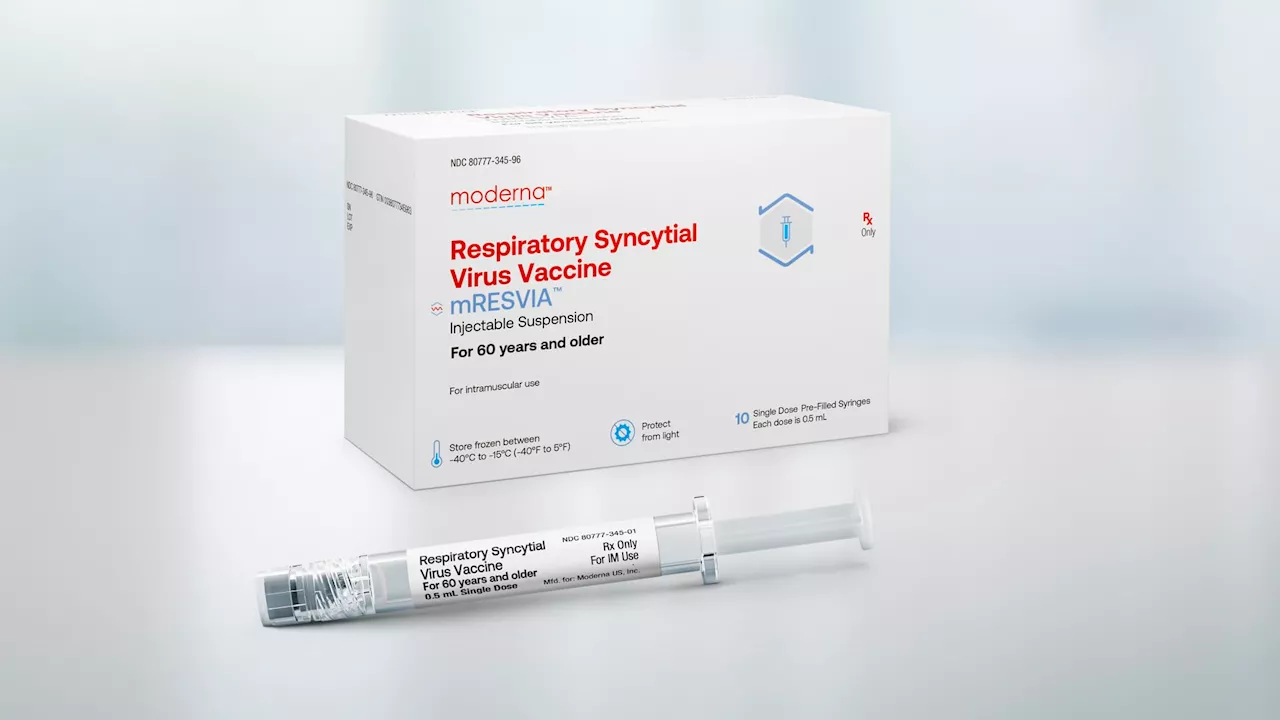 FDA approves Moderna's RSV vaccine for seniors, the company's second-ever productThe decision is a win for Moderna, which needs another revenue source amid plunging demand for its Covid vaccine, its only commercially available product.
FDA approves Moderna's RSV vaccine for seniors, the company's second-ever productThe decision is a win for Moderna, which needs another revenue source amid plunging demand for its Covid vaccine, its only commercially available product.
Read more »
 Moderna says FDA delayed RSV vaccine approval to end of MayModerna on Friday said the Food and Drug Administration has delayed the approval of its vaccine for respiratory syncytial virus to the end of M...
Moderna says FDA delayed RSV vaccine approval to end of MayModerna on Friday said the Food and Drug Administration has delayed the approval of its vaccine for respiratory syncytial virus to the end of M...
Read more »
 Moderna says FDA delayed RSV vaccine approval to end of MayThe FDA has not informed Moderna of any issues related to the vaccine's safety, efficacy or quality that would prevent its approval, the biotech company said.
Moderna says FDA delayed RSV vaccine approval to end of MayThe FDA has not informed Moderna of any issues related to the vaccine's safety, efficacy or quality that would prevent its approval, the biotech company said.
Read more »
 Moderna says FDA delayed RSV vaccine approval to end of MayThe FDA has not informed Moderna of any issues related to the vaccine’s safety, efficacy or quality that would prevent its approval, the biotech company…
Moderna says FDA delayed RSV vaccine approval to end of MayThe FDA has not informed Moderna of any issues related to the vaccine’s safety, efficacy or quality that would prevent its approval, the biotech company…
Read more »
