The FDA has just approved a new injectable treatment for adults with advanced Parkinson’s disease called Vyalev (also known as Produodopa).
Oct. 18, 2024 -- The FDA has approved a new injectable treatment for adults with advanced Parkinson’s disease called Vyalev . This new option is designed to help people who are having trouble managing their symptoms with traditional medications or surgery., a chemical that helps control things like movement, memory, and mood. Without enough dopamine, people with Parkinson’s experience symptoms like tremors, slow movements, stiff muscles, and problems with balance.
This new treatment offers an alternative to oral medications, which can become less effective as Parkinson’s progresses. Robert Hauser, MD, aand Parkinson’s specialist, said that Vyalev gives patients a way to manage their symptoms around the clock without needing surgery.The FDA approval of Vyalev was based on a study of 130 adults with advanced Parkinson’s in the U.S. and Australia.
Most patients saw improvement in their symptoms during the first week of treatment, and those improvements continued for the length of the 12-week study.Like most medications, Vyalev can cause side effects. The most common ones include skin reactions where the injection is given, involuntary movements , and hallucinations .
AbbVie also conducted a longer study to see how safe and effective Vyalev is over time, and the results from that 52-week trial are promising.
United States Latest News, United States Headlines
Similar News:You can also read news stories similar to this one that we have collected from other news sources.
 FDA approves 1st new drug to treat schizophrenia in more than 30 yearsThe FDA on Thursday approved the first new drug to treat patients with schizophrenia in more than 30 years.
FDA approves 1st new drug to treat schizophrenia in more than 30 yearsThe FDA on Thursday approved the first new drug to treat patients with schizophrenia in more than 30 years.
Read more »
 FDA Approves First New Drug to Treat Schizophrenia in 30 YearsCobenfy, a combination of xanomeline and trospium chloride, offers a new approach to managing symptoms like hallucinations, delusions, and disorganized thinking.
FDA Approves First New Drug to Treat Schizophrenia in 30 YearsCobenfy, a combination of xanomeline and trospium chloride, offers a new approach to managing symptoms like hallucinations, delusions, and disorganized thinking.
Read more »
 FDA approves first new medication to treat schizophrenia in more than 30 yearsThe new medication targets a different transmitter to reduce side effects that other antipsychotics typically pose.
FDA approves first new medication to treat schizophrenia in more than 30 yearsThe new medication targets a different transmitter to reduce side effects that other antipsychotics typically pose.
Read more »
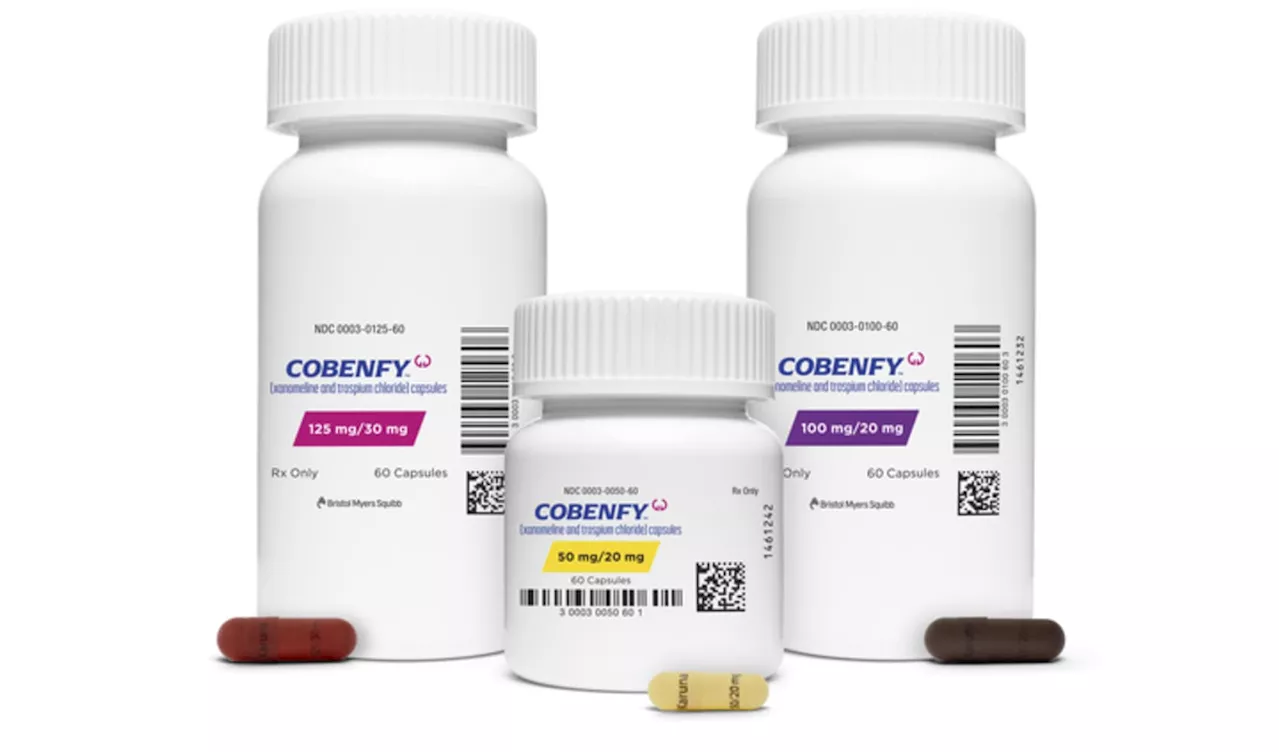 FDA approves Bristol Myers Squibb's schizophrenia drug, the first new type of treatment in decadesThe twice-daily pill, Cobenfy, is a badly needed new treatment option for the nearly 3 million adults in the U.S. living with schizophrenia.
FDA approves Bristol Myers Squibb's schizophrenia drug, the first new type of treatment in decadesThe twice-daily pill, Cobenfy, is a badly needed new treatment option for the nearly 3 million adults in the U.S. living with schizophrenia.
Read more »
 FDA approves 1st new drug for schizophrenia in more than 30 yearsThe medication, Cobenfy, combines two drugs and is taken as a twice-daily pill.
FDA approves 1st new drug for schizophrenia in more than 30 yearsThe medication, Cobenfy, combines two drugs and is taken as a twice-daily pill.
Read more »
 FDA Approves First New Schizophrenia Drug in Over 30 YearsThe FDA has approved Cobenfy, a new drug developed by Bristol Myers Squibb, to treat schizophrenia. This marks the first new treatment for the condition in over three decades.
FDA Approves First New Schizophrenia Drug in Over 30 YearsThe FDA has approved Cobenfy, a new drug developed by Bristol Myers Squibb, to treat schizophrenia. This marks the first new treatment for the condition in over three decades.
Read more »