The FDA has approved Cobenfy, a new drug developed by Bristol Myers Squibb, to treat schizophrenia. This marks the first new treatment for the condition in over three decades.
Dr. Jake Goodman and Liz NeporentThe U.S. Food and Drug Administration on Thursday approved the first new drug to treat people with schizophrenia in more than 30 years.
Cobenfy offers new hope for people with schizophrenia, providing an innovative treatment option that could change how this condition is managed, according to Jelena Kunovac, MD, a board-certified psychiatrist and adjunct assistant professor at the University of Nevada, Las Vegas, in the Department of Psychiatry.
Most schizophrenia medications, broadly known as antipsychotics, work by changing dopamine levels, a brain chemical that affects mood, motivation, and thinking, Kunovac explained. Cobenfy takes a different approach by adjusting acetylcholine, another brain chemical that aids memory, learning and attention, she said.
The most common side effects of Cobenfy are nausea, indigestion, constipation, vomiting, hypertension, abdominal pain, diarrhea, increased heart rate, dizziness and gastroesophageal reflux disease, according to the FDA announcement. Schizophrenia is a mental health disorder that affects about 24 million people worldwide, or roughly one in 300 people,. It often leads to significant challenges in daily functioning, work, and relationships, impacting both patients and their families.
Schizophrenia Drug Approval FDA Cobenfy Mental Health
United States Latest News, United States Headlines
Similar News:You can also read news stories similar to this one that we have collected from other news sources.
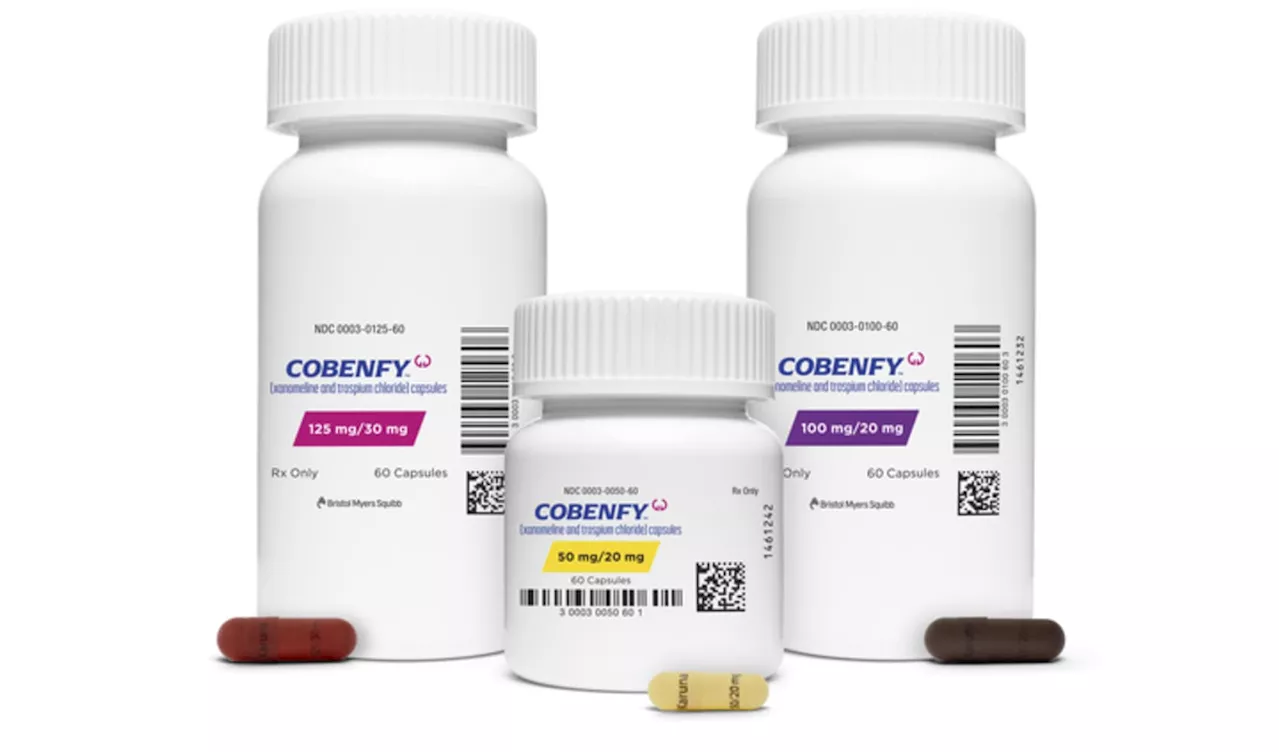 FDA approves Bristol Myers Squibb's schizophrenia drug, the first new type of treatment in decadesThe twice-daily pill, Cobenfy, is a badly needed new treatment option for the nearly 3 million adults in the U.S. living with schizophrenia.
FDA approves Bristol Myers Squibb's schizophrenia drug, the first new type of treatment in decadesThe twice-daily pill, Cobenfy, is a badly needed new treatment option for the nearly 3 million adults in the U.S. living with schizophrenia.
Read more »
 FDA approves 1st new drug to treat schizophrenia in more than 30 yearsThe FDA on Thursday approved the first new drug to treat patients with schizophrenia in more than 30 years.
FDA approves 1st new drug to treat schizophrenia in more than 30 yearsThe FDA on Thursday approved the first new drug to treat patients with schizophrenia in more than 30 years.
Read more »
 FDA approves first at-home syphilis test to combat U.S. rise in sexually transmitted infectionThe at-home syphilis test, which is available without a prescription, gives results in about 15 minutes. Other at-home syphilis tests currently on the market require consumers to send their sample to a lab, the FDA said.
FDA approves first at-home syphilis test to combat U.S. rise in sexually transmitted infectionThe at-home syphilis test, which is available without a prescription, gives results in about 15 minutes. Other at-home syphilis tests currently on the market require consumers to send their sample to a lab, the FDA said.
Read more »
 FDA Approves Apple's AirPods Pro as First Over-the-Counter Hearing AidsJust days after announcing a new hearing aid feature, Apple has received FDA authorization for the software powering this functionality. The FDA describes it as the first over-the-counter (OTC) hearing aid software device.
FDA Approves Apple's AirPods Pro as First Over-the-Counter Hearing AidsJust days after announcing a new hearing aid feature, Apple has received FDA authorization for the software powering this functionality. The FDA describes it as the first over-the-counter (OTC) hearing aid software device.
Read more »
 FDA Approves Apple AirPods Pro as Hearing Aids in Industry FirstThe Best in Science News and Amazing Breakthroughs
FDA Approves Apple AirPods Pro as Hearing Aids in Industry FirstThe Best in Science News and Amazing Breakthroughs
Read more »
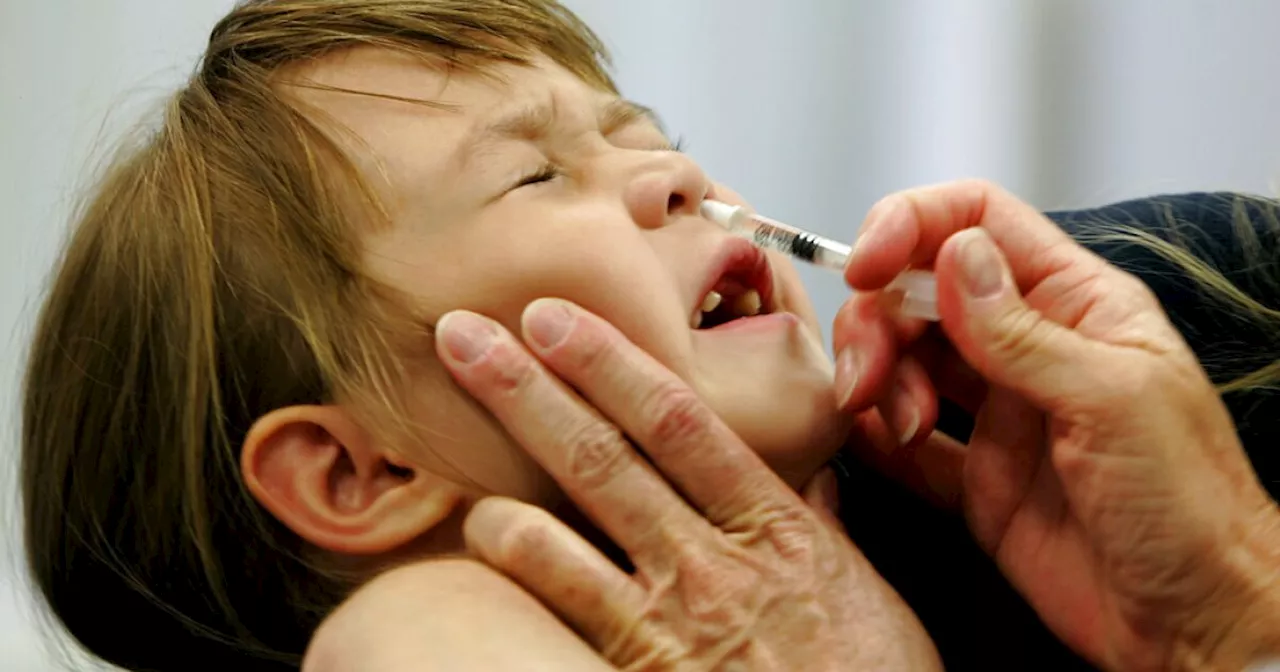 Doctor not included: FDA approves first flu vaccine that can be taken at homeThe Food and Drug Administration announced Friday it has approved the first flu vaccine that individuals can give to themselves at home, without the need for a healthcare professional.
Doctor not included: FDA approves first flu vaccine that can be taken at homeThe Food and Drug Administration announced Friday it has approved the first flu vaccine that individuals can give to themselves at home, without the need for a healthcare professional.
Read more »
