Officials say the vaccine against the deadly virus could be rolled out for older adults by August.
The US Food and Drug Administration has approved a vaccine against respiratory syncytial virus - an illness that kills thousands of Americans each year.
Officials say the vaccine, named Arexvy by the manufacturer GSK, is a major breakthrough that will save many lives."Today's approval of the first RSV vaccine is an important public health achievement to prevent a disease which can be life-threatening," said Dr Peter Marks, who leads the Center for Biologics Evaluation and Research at the Food and Drug Administration .
It kills on an average 100 to 300 children under the age of 5 in the US every year, according to the CDC. In severe cases it can cause bronchiolitis, which includes a build-up of inflammation in the lungs and trouble in breathing.
United States Latest News, United States Headlines
Similar News:You can also read news stories similar to this one that we have collected from other news sources.
 FDA approves world's first RSV vaccine, a shot for adults ages 60 and upBREAKING: FDA approves the world's first respiratory syncytial virus, or RSV, vaccine made by pharmaceutical giant GSK.
FDA approves world's first RSV vaccine, a shot for adults ages 60 and upBREAKING: FDA approves the world's first respiratory syncytial virus, or RSV, vaccine made by pharmaceutical giant GSK.
Read more »
FDA approves world’s first RSV vaccine, a shot for older adultsIn a late-stage clinical trial, the single-dose shot lowered the risk of symptomatic illness by 83% and of severe illness by 94%.
Read more »
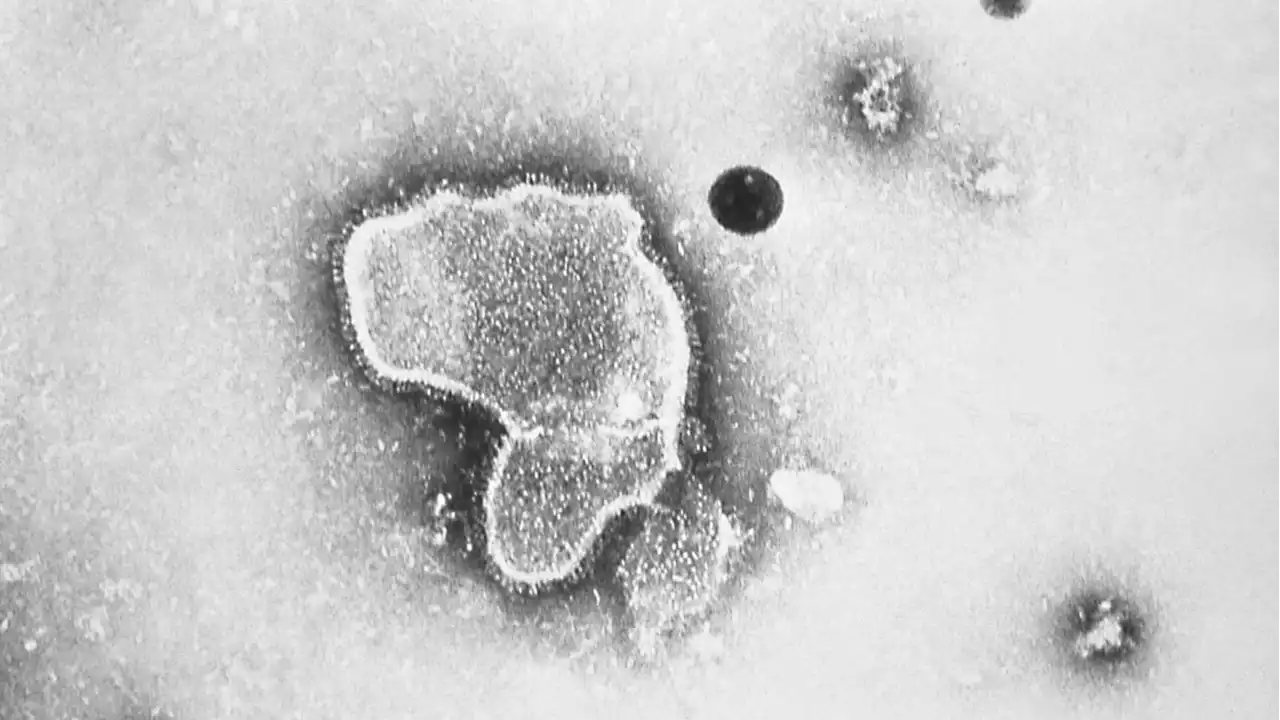 FDA Approves World’s First RSV Vaccine for Adults 60 and OlderResearchers first began attempting to develop a vaccine for respiratory syncytial virus, which is of particular danger to older people and infants, around 60 years ago.
FDA Approves World’s First RSV Vaccine for Adults 60 and OlderResearchers first began attempting to develop a vaccine for respiratory syncytial virus, which is of particular danger to older people and infants, around 60 years ago.
Read more »
RSV in First Year Could Raise Baby’s Risk of AsthmaChildren who didn’t contract RSV in their first year of life had a 26 percent lower risk of asthma later in life. Learn more about the connection here.
Read more »
