The U.S. health regulator on Wednesday approved TG Therapeutics Inc's drug for patients with relapsing forms of multiple sclerosis, pitting it against rivals from Roche and Novartis .
said on Wednesday that the U.S. health regulator had approved its monoclonal antibody for treating patients with relapsing forms of multiple sclerosis, sending its shares up nearly 9% in afternoon trade.
TG Therapeutics said it was expecting to launch the drug, branded as Brumvi, in the first quarter of 2023, but did not give details on its pricing. Multiple sclerosis is a neurological disease in which the immune system attacks the brain cells causing motor disabilities. It affects about 400,000 people in the United States, according to the National Institutes of Health.
United States Latest News, United States Headlines
Similar News:You can also read news stories similar to this one that we have collected from other news sources.
 Jounce stock bounces more than 70% after Gilead acquires immunotherapy drugJounce Therapeutics Inc. shares jumped more than 70% in after-hours trading Tuesday, following an announcement that Gilead Sciences Inc. would completely...
Jounce stock bounces more than 70% after Gilead acquires immunotherapy drugJounce Therapeutics Inc. shares jumped more than 70% in after-hours trading Tuesday, following an announcement that Gilead Sciences Inc. would completely...
Read more »
 Challenger, Gray & Christmas, Inc. Job Hotline Offering Free Career CounsellingA new job may be just a phone call away. Career consultants from coaching company Challenger, Gray & Christmas, Inc. are accepting free calls Tuesday and Wednesday. NBC 5’s Kye Martin reports.
Challenger, Gray & Christmas, Inc. Job Hotline Offering Free Career CounsellingA new job may be just a phone call away. Career consultants from coaching company Challenger, Gray & Christmas, Inc. are accepting free calls Tuesday and Wednesday. NBC 5’s Kye Martin reports.
Read more »
 AMC Stock Price | AMC Entertainment Holdings Inc. Cl A Stock Quote (U.S.: NYSE) | MarketWatchAMC | Complete AMC Entertainment Holdings Inc. Cl A stock news by MarketWatch. View real-time stock prices and stock quotes for a full financial overview.
AMC Stock Price | AMC Entertainment Holdings Inc. Cl A Stock Quote (U.S.: NYSE) | MarketWatchAMC | Complete AMC Entertainment Holdings Inc. Cl A stock news by MarketWatch. View real-time stock prices and stock quotes for a full financial overview.
Read more »
 Kala Pharmaceuticals stock rockets after FDA accepts IND application for PCED treatmentShares of Kala Pharmaceuticals Inc. shot up 45.7% in premarket trading Wednesday, after the biopharmaceutical company said the Food and Drug Administration...
Kala Pharmaceuticals stock rockets after FDA accepts IND application for PCED treatmentShares of Kala Pharmaceuticals Inc. shot up 45.7% in premarket trading Wednesday, after the biopharmaceutical company said the Food and Drug Administration...
Read more »
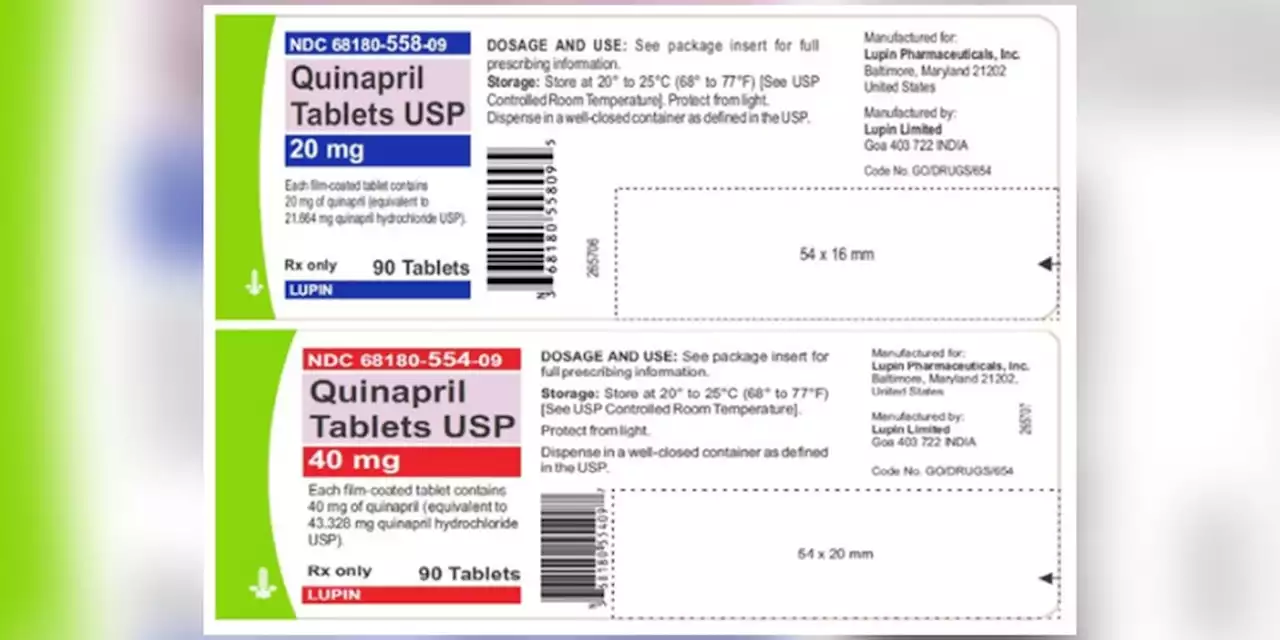 Blood pressure tablets recalled due to potential cancer risks, FDA saysLupin Pharmaceuticals Inc. issued the recall for four lots of Quinapril 20 mg and 40 mg tablets due to the presence of a nitrosamine that is above the recommended daily intake.
Blood pressure tablets recalled due to potential cancer risks, FDA saysLupin Pharmaceuticals Inc. issued the recall for four lots of Quinapril 20 mg and 40 mg tablets due to the presence of a nitrosamine that is above the recommended daily intake.
Read more »
 Blood pressure tablets recalled due to potential cancer risks, FDA saysLupin Pharmaceuticals Inc. issued the recall for four lots of Quinapril 20 mg and 40 mg tablets due to the presence of a nitrosamine that is above the recommended daily intake.
Blood pressure tablets recalled due to potential cancer risks, FDA saysLupin Pharmaceuticals Inc. issued the recall for four lots of Quinapril 20 mg and 40 mg tablets due to the presence of a nitrosamine that is above the recommended daily intake.
Read more »
