But you’ll have to wait until One UI 5 Watch arrives later this year.
Overall, the new feature slots nicely into Samsung’s goal to position the Galaxy Watch devices as more holistic health tools. That said, it’s clear that Samsung is still playing a bit of catch-up.
Most of the One UI 5 Watch updates — like personalized heart rate zones and improved emergency SOS — are attempts to close the gap with other smartwatch makers. The same holds true here. As mentioned, Fitbit introduced a similar feature last year. Apple also introduced an with watchOS 9, which allows people with irregular heart rate rhythms to track the amount of time they spend in AFib.
United States Latest News, United States Headlines
Similar News:You can also read news stories similar to this one that we have collected from other news sources.
 Column: Washington star Trinity Rodman's one-goal, one-assist performance leaves Wave dispiritedDennis Rodman's daughter, the NWSL's highest-paid player, strengthens World Cup bid in Spirit's win over the Wave; Alex Morgan scores for San Diego
Column: Washington star Trinity Rodman's one-goal, one-assist performance leaves Wave dispiritedDennis Rodman's daughter, the NWSL's highest-paid player, strengthens World Cup bid in Spirit's win over the Wave; Alex Morgan scores for San Diego
Read more »
 Katy Perry Responds After Viral Coronation Seat Confusion“That was one of the most relatable moments of the Coronation,” one fan said on Twitter.
Katy Perry Responds After Viral Coronation Seat Confusion“That was one of the most relatable moments of the Coronation,” one fan said on Twitter.
Read more »
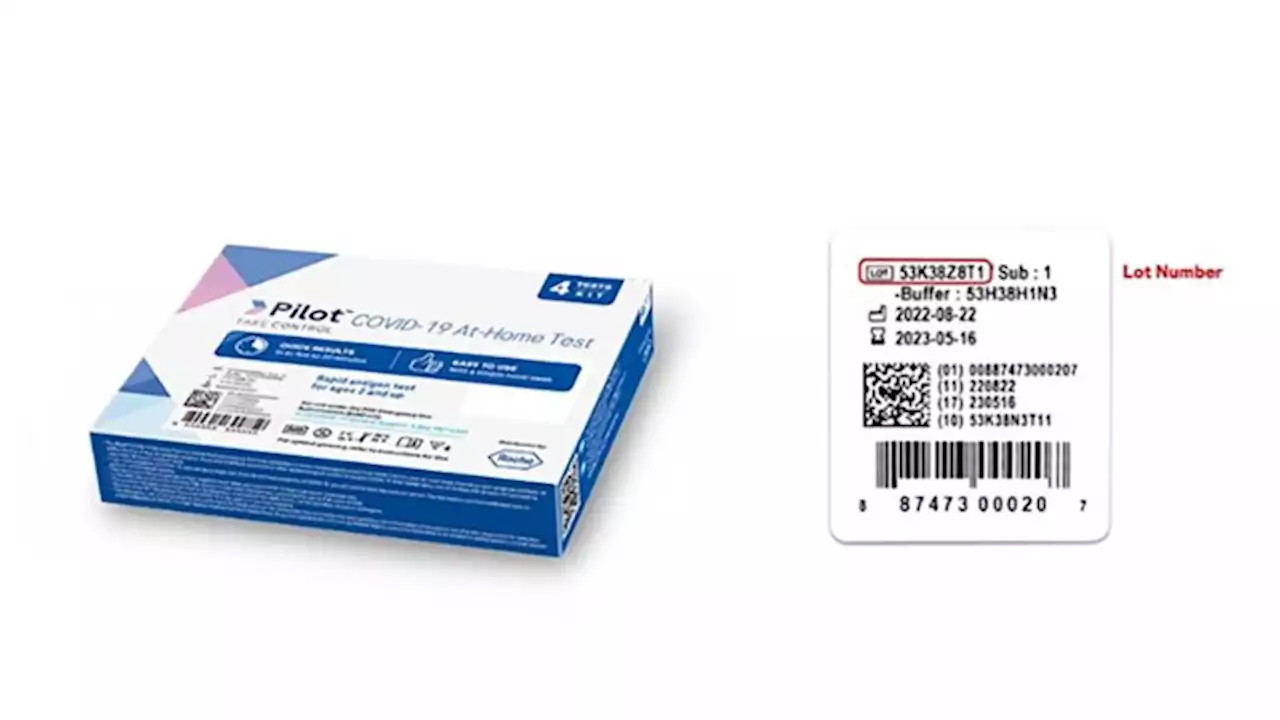 Some Pilot COVID-19 At Home Tests recalled by FDA over bacteria risk | CNNThe US Food and Drug Administration issued a warning on Friday to consumers and health providers to discontinue using and discard recalled Pilot COVID-19 At-Home Tests made by SD Biosensor, Inc. over 'significant concerns' of bacterial contamination.
Some Pilot COVID-19 At Home Tests recalled by FDA over bacteria risk | CNNThe US Food and Drug Administration issued a warning on Friday to consumers and health providers to discontinue using and discard recalled Pilot COVID-19 At-Home Tests made by SD Biosensor, Inc. over 'significant concerns' of bacterial contamination.
Read more »
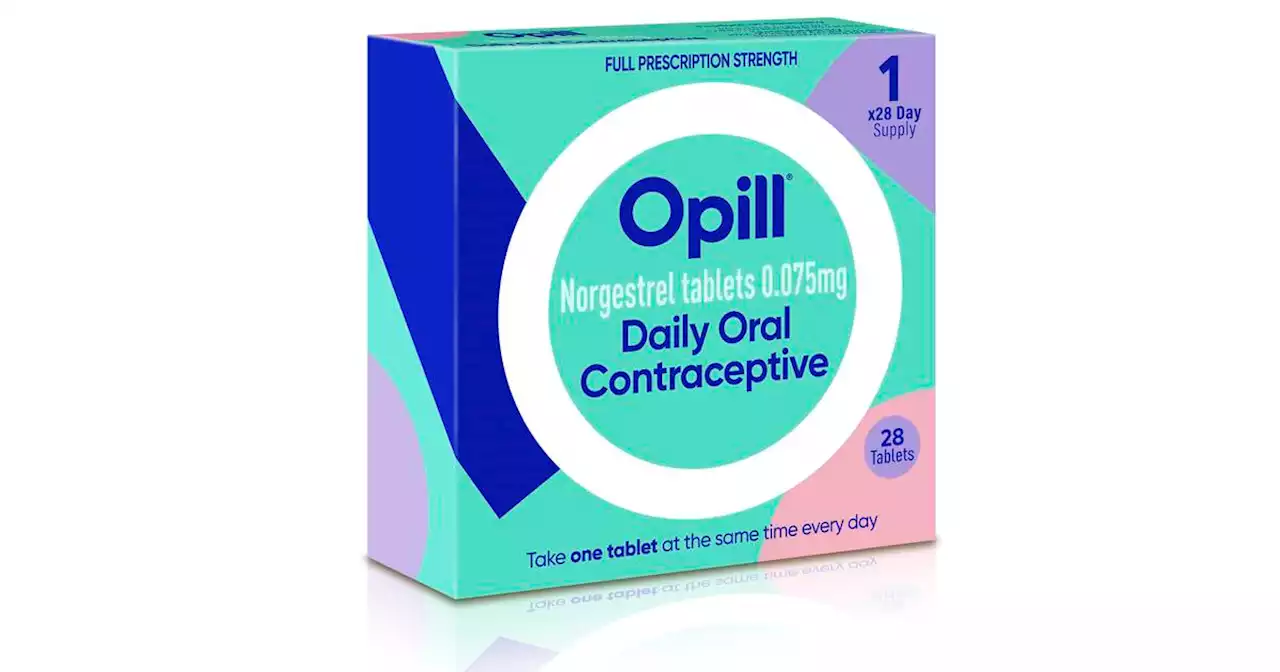 FDA weighing first over-the-counter birth control pillOn Friday, the Food and Drug Administration posted its initial review of a decades-old pill that could become the first drug of its kind to move over the counter.
FDA weighing first over-the-counter birth control pillOn Friday, the Food and Drug Administration posted its initial review of a decades-old pill that could become the first drug of its kind to move over the counter.
Read more »
 Immuron stock rockets after FDA removes hold on IND of infectious diarrhea treatmentThe U.S.-listed shares of Immuron Ltd. powered up 66.3% toward a one-year high in premarket trading Monday, after the Australia-based biopharmaceutical...
Immuron stock rockets after FDA removes hold on IND of infectious diarrhea treatmentThe U.S.-listed shares of Immuron Ltd. powered up 66.3% toward a one-year high in premarket trading Monday, after the Australia-based biopharmaceutical...
Read more »
