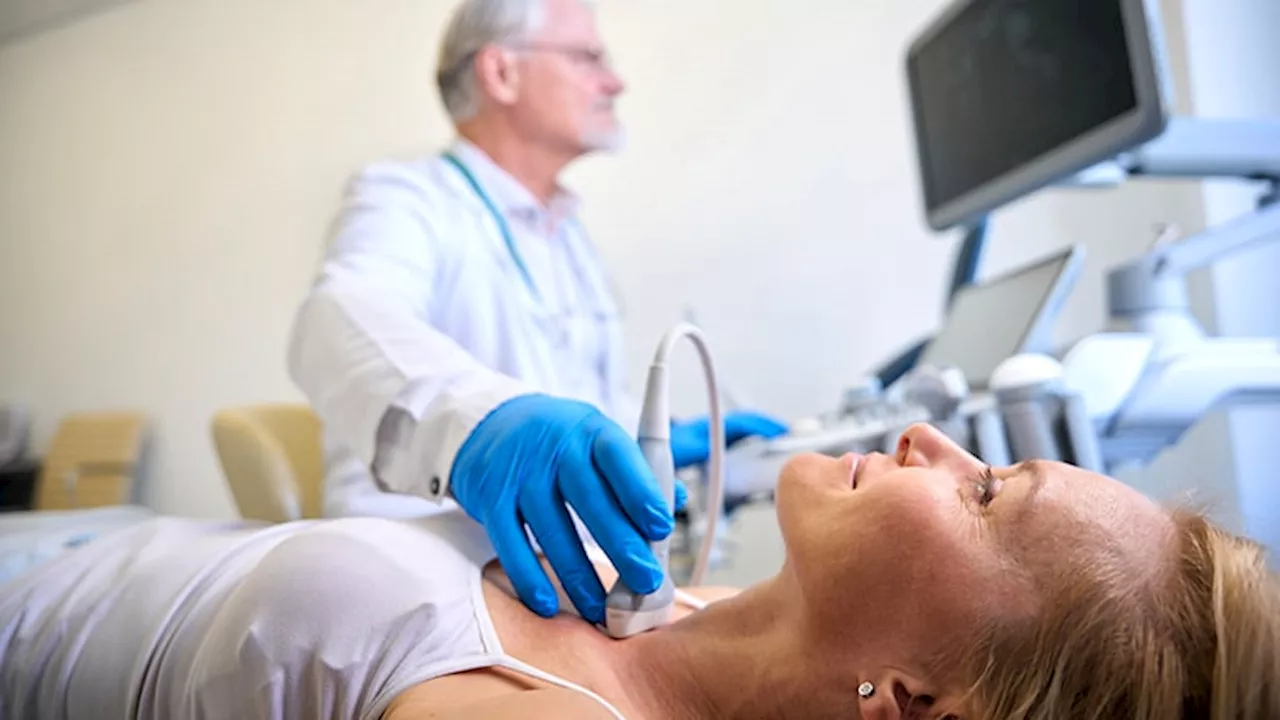
The See-Mode system is said to be the first of its kind in thyroid nodule detection and diagnosis.
The US Food and Drug Administration has issued 510 clearance to See-Mode Technologies for their AI-based thyroid ultrasound analysis and reporting software. While various AI tools for the evaluation of thyroid nodule s have received FDA approval, See-Mode reports that their technology is the first FDA-cleared product providing detection as well as diagnosis for thyroid ultrasound.
The features are designed to reduce reporting time as well as variation in delivery of care for thyroid ultrasound, the company reported.A multireader, multicase study included in the FDA submission demonstrated improvement in radiologist performance with the aid of the technology, See-Mode reported.
Thyroid Disease Thyroid Dysfunction Thyroid Nodule Thyroid Healthcare And Medical Technology Health And Medical Tech Health And Med Tech Health And Medical Technology Healthcare Technology Medical Technology Artificial Intelligence Deep Learning AI NPL Machine Learning ML Natural Language Processing Artificial Neural Networks Ultrasonography Ultrasound Sonogram
United States Latest News, United States Headlines
Similar News:You can also read news stories similar to this one that we have collected from other news sources.
 FDA Clears First Automated Device to Deliver Insulin to People With Type 2 DiabetesThe U.S. Food and Drug Administration on Monday approved the expanded use of an automated insulin pump system to include those with type 2 diabetes.
FDA Clears First Automated Device to Deliver Insulin to People With Type 2 DiabetesThe U.S. Food and Drug Administration on Monday approved the expanded use of an automated insulin pump system to include those with type 2 diabetes.
Read more »
 FDA Clears Apple Watch For Sleep Apnea DetectionThe U.S. Food and Drug Administration (FDA) has granted approval for the Apple Watch to detect sleep apnea, recognizing its accuracy and reliability based on Apple's approach being 'substantially equivalent' to established methods. The watch utilizes its built-in accelerometer to track breathing patterns during sleep and alert users to consult a healthcare provider.
FDA Clears Apple Watch For Sleep Apnea DetectionThe U.S. Food and Drug Administration (FDA) has granted approval for the Apple Watch to detect sleep apnea, recognizing its accuracy and reliability based on Apple's approach being 'substantially equivalent' to established methods. The watch utilizes its built-in accelerometer to track breathing patterns during sleep and alert users to consult a healthcare provider.
Read more »
 FDA Clears Apple’s AirPods Pro to Act as Your Hearing AidsApple’s $250 AirPods Pro 2 aren’t cheap, but they're a fraction of what you would pay for even a cheap pair of OTC hearing aids.
FDA Clears Apple’s AirPods Pro to Act as Your Hearing AidsApple’s $250 AirPods Pro 2 aren’t cheap, but they're a fraction of what you would pay for even a cheap pair of OTC hearing aids.
Read more »
 FDA Clears the Omnipod 5 System for Type 2 DiabetesNew indication represents the first for use of an automated insulin delivery system in people with type 2 diabetes who require insulin treatment.
FDA Clears the Omnipod 5 System for Type 2 DiabetesNew indication represents the first for use of an automated insulin delivery system in people with type 2 diabetes who require insulin treatment.
Read more »
 Lobectomy Doesn't Raise Reoperation Rates in Thyroid CancerDespite a shift toward performing more initial lobectomies for thyroid cancer following the release of the 2015 ATA guidelines, the rate of late reoperations remained unchanged.
Lobectomy Doesn't Raise Reoperation Rates in Thyroid CancerDespite a shift toward performing more initial lobectomies for thyroid cancer following the release of the 2015 ATA guidelines, the rate of late reoperations remained unchanged.
Read more »
 Thyroid Surgery Increases Kidney Disease RiskPatients who underwent total thyroidectomy had an increased risk for chronic kidney disease, particularly those who developed hypoparathyroidism.
Thyroid Surgery Increases Kidney Disease RiskPatients who underwent total thyroidectomy had an increased risk for chronic kidney disease, particularly those who developed hypoparathyroidism.
Read more »