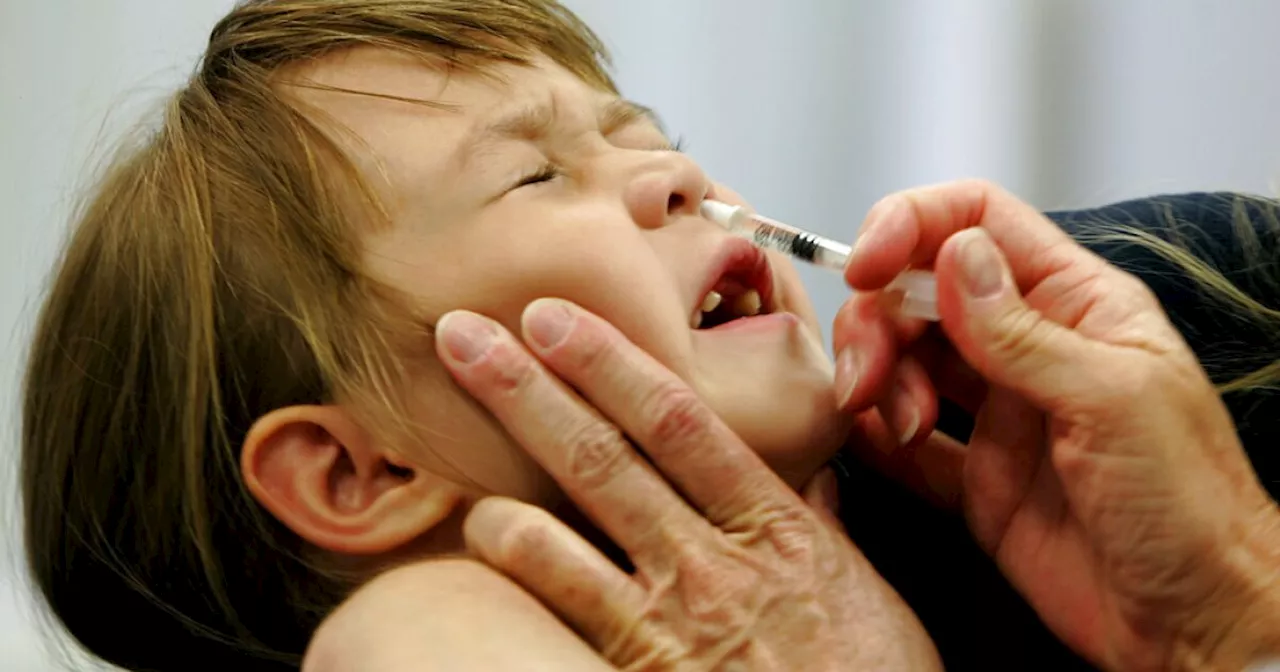
The Food and Drug Administration (FDA) has approved FluMist, a nasal spray flu vaccine, allowing individuals to administer it at home without a healthcare professional. Users complete a screening and eligibility assessment through an online pharmacy, receive the vaccine by mail with instructions, and can choose to have it administered by a caregiver for children.
The Food and Drug Administration announced Friday it has approved the first flu vaccine that individuals can give to themselves at home without the need for a healthcare professional.FluMist, a nasal spray, has been approved to prevent influenza from both A and B viruses in almost all ages since 2007.Now, you won’t need to go to a doctor or pharmacy to get it administered — although you still can if you prefer to.
Once approved, the prescription will be shipped to your address with instructions on how to use it.The FDA said a caregiver should administer the FluMist vaccine to children.The nasal spray vaccine contains a weakened form of live flu strains. The most commonly reported side effects, the FDA said, are a fever in young children, runny nose, nasal congestion and/or a sore throat.According to the U.S. Centers for Disease Control and Prevention, flu has resulted in about 9.
Fluvaccine Fdaapproval Selfadministered Flumist Nasalspray
United States Latest News, United States Headlines
Similar News:You can also read news stories similar to this one that we have collected from other news sources.
 Weight-loss drug safe, effective for kids as young as 6, study saysSaxenda was the FDA's first approved drug to treat obesity in adults back in 2014.
Weight-loss drug safe, effective for kids as young as 6, study saysSaxenda was the FDA's first approved drug to treat obesity in adults back in 2014.
Read more »
 Nearly 2,000 drug manufacturing plants are overdue for FDA inspections after COVID delays, AP findsU.S. health inspectors are still struggling to address a massive backlog of pharmaceutical plants that went uninspected during disruptions caused by COVID-19. That's according to an analysis of government data by the Associated Press.
Nearly 2,000 drug manufacturing plants are overdue for FDA inspections after COVID delays, AP findsU.S. health inspectors are still struggling to address a massive backlog of pharmaceutical plants that went uninspected during disruptions caused by COVID-19. That's according to an analysis of government data by the Associated Press.
Read more »
 Nearly 2,000 drug manufacturing plants are overdue for FDA inspections after COVID delays, AP findsWASHINGTON (AP) — Federal regulators responsible for the safety of the U.S. drug supply are still struggling to get back to where they were in 2019,
Nearly 2,000 drug manufacturing plants are overdue for FDA inspections after COVID delays, AP findsWASHINGTON (AP) — Federal regulators responsible for the safety of the U.S. drug supply are still struggling to get back to where they were in 2019,
Read more »
 Nearly 2,000 drug plants are overdue for FDA checks after COVID delays, AP findsU.S. health inspectors are still struggling to address a massive backlog of pharmaceutical plants that went uninspected during disruptions caused by COVID-19.
Nearly 2,000 drug plants are overdue for FDA checks after COVID delays, AP findsU.S. health inspectors are still struggling to address a massive backlog of pharmaceutical plants that went uninspected during disruptions caused by COVID-19.
Read more »
 Nearly 2,000 drug plants are overdue for FDA checks after COVID delays, AP findsFederal regulators responsible for the safety of the U.S. drug supply are still struggling to get back to where they were in 2019, before the COVID-19 pandemic…
Nearly 2,000 drug plants are overdue for FDA checks after COVID delays, AP findsFederal regulators responsible for the safety of the U.S. drug supply are still struggling to get back to where they were in 2019, before the COVID-19 pandemic…
Read more »
 Nearly 2,000 drug plants are overdue for FDA checks after COVID delays, AP findsFederal regulators responsible for the safety of the U.S. drug supply are still struggling to get back to where they were in 2019, before the COVID-19 pandemic…
Nearly 2,000 drug plants are overdue for FDA checks after COVID delays, AP findsFederal regulators responsible for the safety of the U.S. drug supply are still struggling to get back to where they were in 2019, before the COVID-19 pandemic…
Read more »