Ciara Linnane is MarketWatch's investing- and corporate-news editor. She is based in New York.
Crispr Therapeutics AG’s stock CRSP, +0.49% rallied 17% in premarket trade Wednesday, after a positive meeting of a . Food and Drug Administration advisory panel on the gene-editing drug exa-cel developed by Crispr and partner Vertex Pharmaceuticals VRTX, +1.30% as a treatment for sickle-cell disease. The FDA has set a Dec. 8 target action date for possible approval of the treatment, which would be the first approved therapy that uses the gene-editing technology called Crispr.
“Rational thinking prevailed at today’s FDA ad com,” wrote analysts at Evercore ISI, in a note. “We think exa-cel is very likely to be approved by December 8.”The analysts described it as a “clearing event” for Vertex and a “nice win for CRSP, their platform, and important medical advancement for the field and for patients.” Sickle-cell disease is a painful inherited blood disorder that affects an estimated 70,000 to 100,000 Americans a year, according to the American Society of Hematology.
United States Latest News, United States Headlines
Similar News:You can also read news stories similar to this one that we have collected from other news sources.
 FDA Advisors Consider Crispr Gene-Editing Treatment for Sickle Cell AnemiaNearly all patients treated so far have been relieved of the blood-clogging crises of the disease.
FDA Advisors Consider Crispr Gene-Editing Treatment for Sickle Cell AnemiaNearly all patients treated so far have been relieved of the blood-clogging crises of the disease.
Read more »
 FDA Advisors Consider Crispr Gene-Editing Treatment for Sickle Cell AnemiaNearly all patients treated so far have been relieved of the blood-clogging crises of the disease.
FDA Advisors Consider Crispr Gene-Editing Treatment for Sickle Cell AnemiaNearly all patients treated so far have been relieved of the blood-clogging crises of the disease.
Read more »
 FDA panel says Vertex/CRISPR to assess safety risks of gene therapy in follow-up studyA panel of advisers to the U.S. health regulator said on Tuesday Vertex Pharmaceuticals (VRTX.O) and CRISPR Therapeutics (CRSP.BN) could assess potential safety risks of their sickle cell disease gene therapy after approval.
FDA panel says Vertex/CRISPR to assess safety risks of gene therapy in follow-up studyA panel of advisers to the U.S. health regulator said on Tuesday Vertex Pharmaceuticals (VRTX.O) and CRISPR Therapeutics (CRSP.BN) could assess potential safety risks of their sickle cell disease gene therapy after approval.
Read more »
 FDA panel weighs CRISPR-based sickle cell therapyThe FDA must decide whether to approve the therapy by Dec. 8.
FDA panel weighs CRISPR-based sickle cell therapyThe FDA must decide whether to approve the therapy by Dec. 8.
Read more »
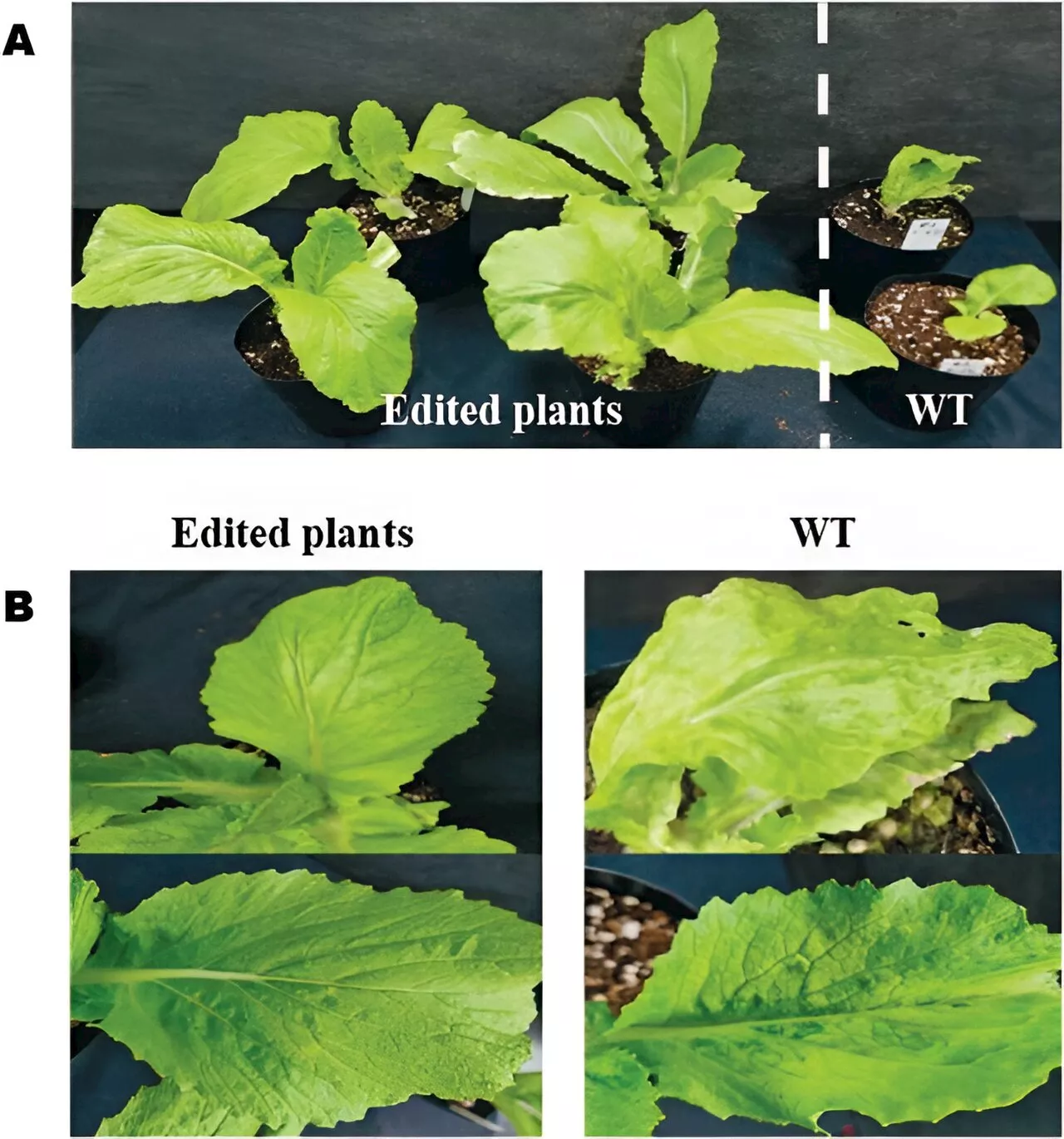 CRISPR/Cas9 unlocks TuMV resistance in Chinese cabbage: A leap forward in genome-edited plant breedingGenome editing, especially the CRISPR/Cas9 technology, holds immense promise in enhancing plant traits, primarily disease resistance, offering a more efficient alternative to traditional breeding.
CRISPR/Cas9 unlocks TuMV resistance in Chinese cabbage: A leap forward in genome-edited plant breedingGenome editing, especially the CRISPR/Cas9 technology, holds immense promise in enhancing plant traits, primarily disease resistance, offering a more efficient alternative to traditional breeding.
Read more »
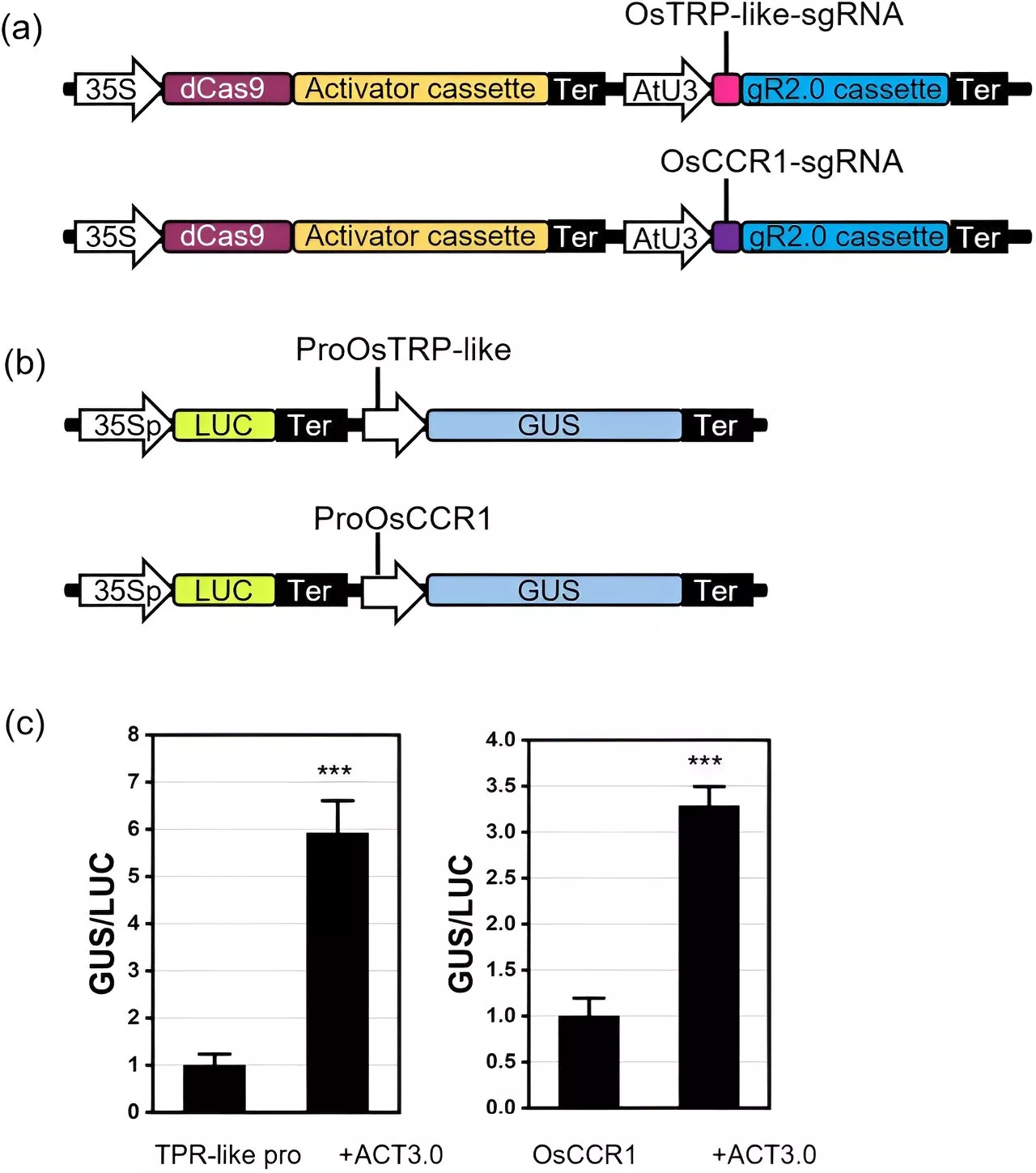 Unlocking the genetic potential of poplars: A new era of precision genome editing with CRISPRThe Populus genus, commonly known as poplars, cottonwoods, and aspens, consists of approximately 30 tree species native to the northern hemisphere. Because of their diverse usages in landscape, agriculture, bioenergy, and industry, Populus species have been the focus of many tree breeding and genetic improvement programs.
Unlocking the genetic potential of poplars: A new era of precision genome editing with CRISPRThe Populus genus, commonly known as poplars, cottonwoods, and aspens, consists of approximately 30 tree species native to the northern hemisphere. Because of their diverse usages in landscape, agriculture, bioenergy, and industry, Populus species have been the focus of many tree breeding and genetic improvement programs.
Read more »