Researchers have published results of a phase I clinical trial of a novel immunotherapy for high-risk sarcomas.
CAR T cell therapy targeting HER2 antigen shows promise against advanced sarcoma in phase I trial." ScienceDaily. ScienceDaily, 24 April 2024. <www.sciencedaily.comBaylor College of Medicine. . CAR T cell therapy targeting HER2 antigen shows promise against advanced sarcoma in phase I trial.Baylor College of Medicine."CAR T cell therapy targeting HER2 antigen shows promise against advanced sarcoma in phase I trial." ScienceDaily. www.sciencedaily.
A collaboration of researchers has published successful results from a Phase II clinical trial for the treatment of BRAF mutated low-grade paediatric ... A global phase 3 clinical trial found that a year-long immunotherapy through a skin patch safely desensitized toddlers with peanut allergy, lowering the risk of a severe allergic reaction from ...
A recent phase II clinical trial results suggest that the monoclonal antibody hu14.18K322A could help change treatment of children with high-risk ... Combining a histone deacetylase inhibitor drug with immunotherapy agents has been deemed safe, and may benefit some patients with advanced cancers that have not responded to traditional therapy, ...LIVING & WELL
Lung Cancer Brain Tumor Lymphoma Skin Cancer Colon Cancer Leukemia Breast Cancer
United States Latest News, United States Headlines
Similar News:You can also read news stories similar to this one that we have collected from other news sources.
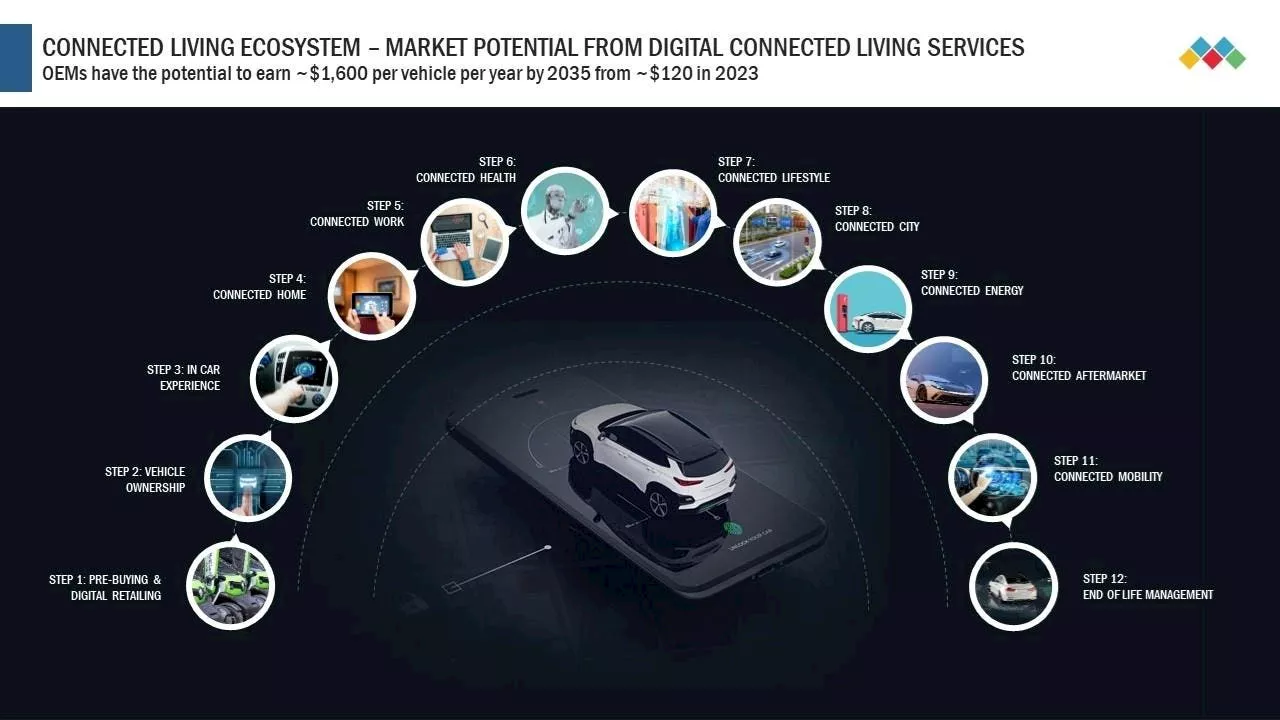 Car Companies Can Generate $1600 Per Car In Future From Connected Car ServicesSarwant has over 29 years of work experience in leading major advisory and transformation engagements focusing on making organizations future ready. Sarwant is currently working with Markets and Markets as President and Chief Commercial office.
Car Companies Can Generate $1600 Per Car In Future From Connected Car ServicesSarwant has over 29 years of work experience in leading major advisory and transformation engagements focusing on making organizations future ready. Sarwant is currently working with Markets and Markets as President and Chief Commercial office.
Read more »
 Researchers identify protein that controls CAR T cell longevityCAR T cell therapy has revolutionized the way certain types of cancer are treated, and the longer those CAR T cells live in a patient's body, the more effectively they respond to cancer.
Researchers identify protein that controls CAR T cell longevityCAR T cell therapy has revolutionized the way certain types of cancer are treated, and the longer those CAR T cells live in a patient's body, the more effectively they respond to cancer.
Read more »
 Monitoring ctDNA Provides Early Response Insights for Targeted Therapies in HER2-Altered CancersA study reveals that monitoring a patient's circulating tumor DNA (ctDNA) can offer valuable insights on early response to targeted therapies in patients with HER2-altered cancers. The findings suggest that ctDNA dynamics can predict treatment response, providing clinicians with important prognostic information.
Monitoring ctDNA Provides Early Response Insights for Targeted Therapies in HER2-Altered CancersA study reveals that monitoring a patient's circulating tumor DNA (ctDNA) can offer valuable insights on early response to targeted therapies in patients with HER2-altered cancers. The findings suggest that ctDNA dynamics can predict treatment response, providing clinicians with important prognostic information.
Read more »
 Monitoring ctDNA Predicts HER2+ Cancer Treatment ResponseTracking a patient's circulating tumor DNA (ctDNA) can offer insight into how well-targeted therapies work in the early stages of treatment for HER2-positive cancers.
Monitoring ctDNA Predicts HER2+ Cancer Treatment ResponseTracking a patient's circulating tumor DNA (ctDNA) can offer insight into how well-targeted therapies work in the early stages of treatment for HER2-positive cancers.
Read more »
 FDA Expands Approval of Fam-Trastuzumab-Deruxtecan-Nxki for HER2-Positive Gastric CancerThe US Food and Drug Administration (FDA) has expanded the approval of fam-trastuzumab–deruxtecan-nxki (Enhertu; AstraZeneca and Daiichi Sankyo, Inc) to adults with unresectable or metastatic as well as adults with locally advanced or metastatic HER2-positive gastric or gastroesophageal junction adenocarcinoma who had received a prior trastuzumab-based regimen. This approval provides a new treatment option for patients with limited options.
FDA Expands Approval of Fam-Trastuzumab-Deruxtecan-Nxki for HER2-Positive Gastric CancerThe US Food and Drug Administration (FDA) has expanded the approval of fam-trastuzumab–deruxtecan-nxki (Enhertu; AstraZeneca and Daiichi Sankyo, Inc) to adults with unresectable or metastatic as well as adults with locally advanced or metastatic HER2-positive gastric or gastroesophageal junction adenocarcinoma who had received a prior trastuzumab-based regimen. This approval provides a new treatment option for patients with limited options.
Read more »
 FDA Expands Enhertu Indication to HER2-Positive Solid TumorsThe move from the FDA marks the first tumor-agnostic approval of a HER2-directed therapy and antibody drug conjugate.
FDA Expands Enhertu Indication to HER2-Positive Solid TumorsThe move from the FDA marks the first tumor-agnostic approval of a HER2-directed therapy and antibody drug conjugate.
Read more »
