The FDA and CDC have signed off on a third COVID shot, which will be a bivalent vaccine, for children. The new shots should be available almost immediately, according to both companies and Walgreens, which is making appointments for next week.
Although the antiviral Paxlovid is believed to remain effective against all the variants, there are no longer any monoclonal antibodies that can reduce disease severity once someone is infected.
"As this virus has changed, and immunity from previous COVID-19 vaccination wanes, the more people who keep up to date on COVID-19 vaccinations, the more benefit there will be for individuals, families and public health by helping prevent severe illnesses, hospitalizations and deaths," FDA Commissioner Dr. Robert Califf said in a prepared statement.The Pfizer-BioNTech vaccine is meant to be given in three doses to children ages 6 months through 4 years old.
United States Latest News, United States Headlines
Similar News:You can also read news stories similar to this one that we have collected from other news sources.
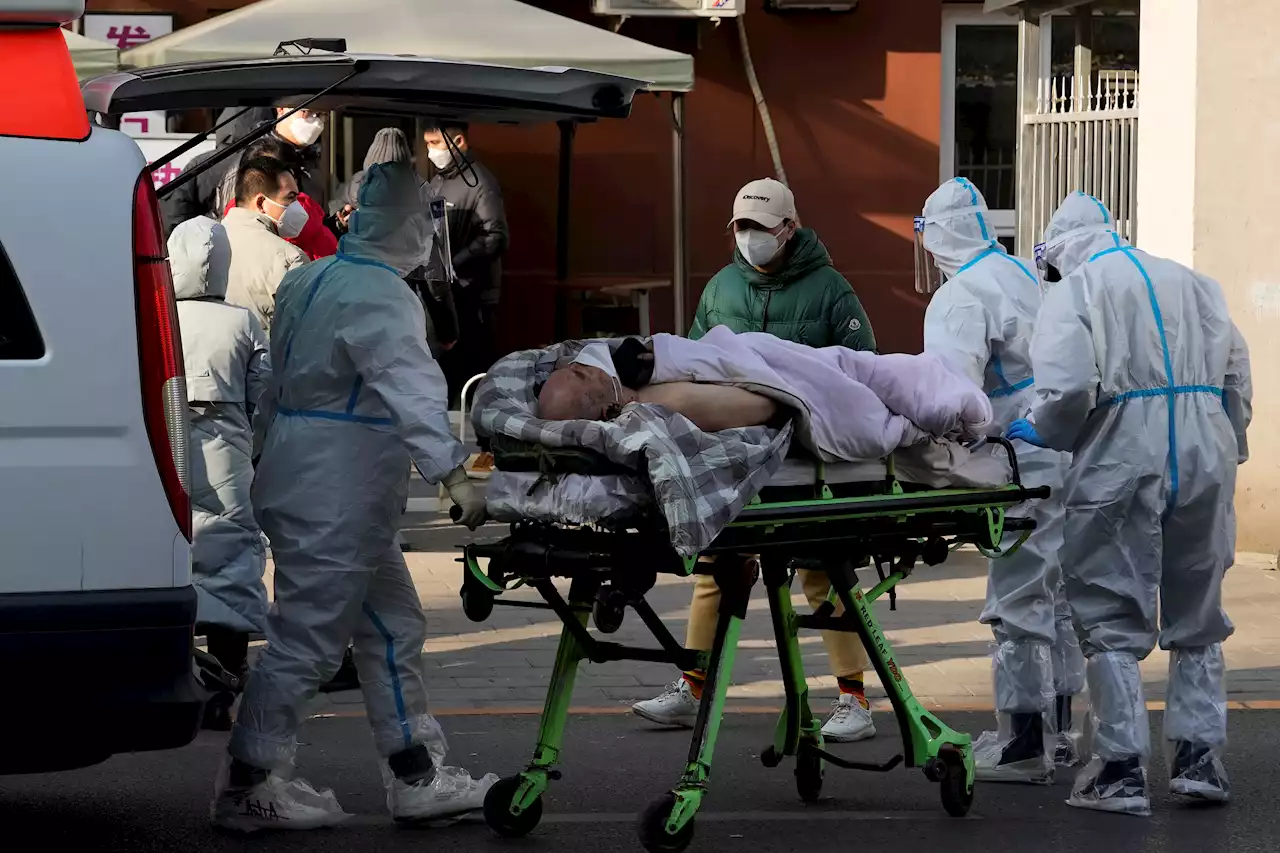 China Struggles With COVID Wave After 'Zero-COVID' EasesA rash of COVID-19 cases in schools and businesses were reported by social media users Friday in areas across China after the ruling Communist Party loosened anti-virus rules as it tries to reverse a deepening economic slump.
China Struggles With COVID Wave After 'Zero-COVID' EasesA rash of COVID-19 cases in schools and businesses were reported by social media users Friday in areas across China after the ruling Communist Party loosened anti-virus rules as it tries to reverse a deepening economic slump.
Read more »
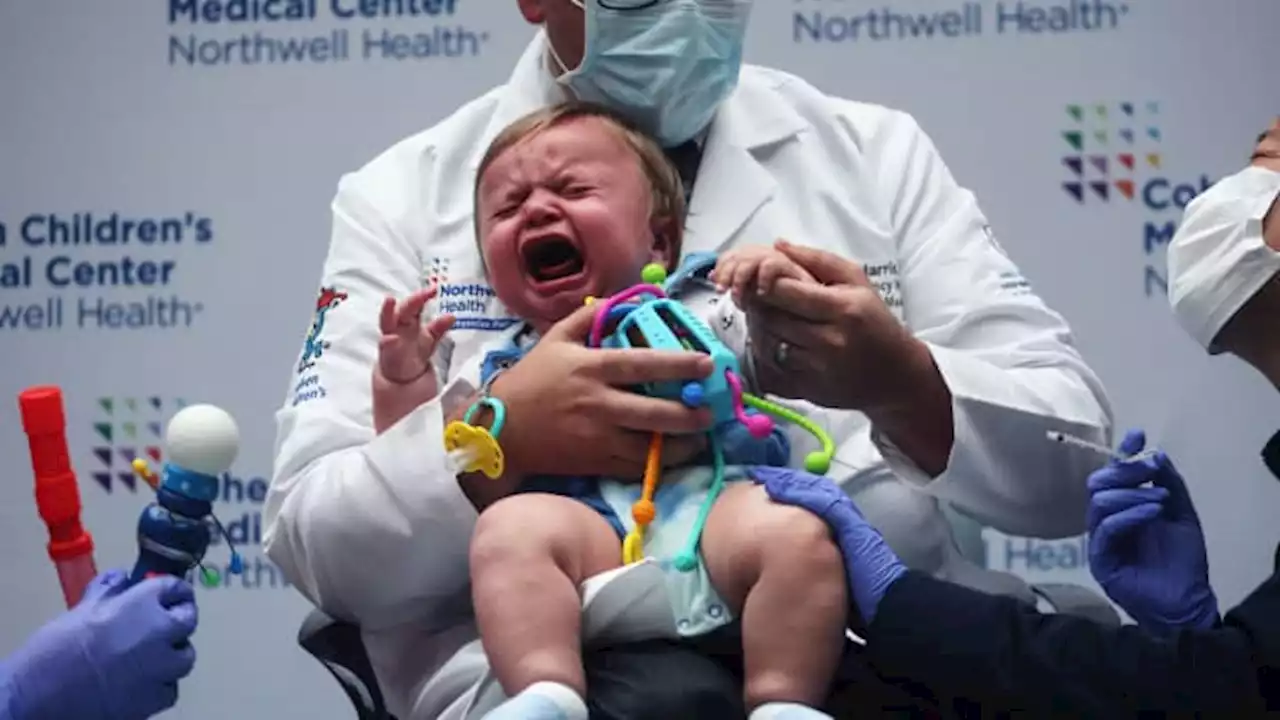 FDA authorizes Covid omicron vaccines for children as young as 6 months old
FDA authorizes Covid omicron vaccines for children as young as 6 months old
Read more »
 FDA authorizes updated COVID shots for kids as young as 6 months oldThe FDA is encouraging families to get young children vaccinated ahead of the holidays.
FDA authorizes updated COVID shots for kids as young as 6 months oldThe FDA is encouraging families to get young children vaccinated ahead of the holidays.
Read more »
 FDA says some young kids can't get an updated COVID booster until next yearThe FDA says it is waiting for data to be submitted next year to clear shots for some of the youngest Pfizer vaccine recipients.
FDA says some young kids can't get an updated COVID booster until next yearThe FDA says it is waiting for data to be submitted next year to clear shots for some of the youngest Pfizer vaccine recipients.
Read more »
